|
|
|
Electrons and stuff.
In about 1975, in my laboratory in a Sydney High School where I was teaching, I was chatting with one of the great old Science teachers of the day. He had retired, was then 75 years of age, but was still occasionally doing some casual relief teaching. I can’t remember how the matter arose, but I suddenly realized that the old teacher believed that electrons moved through a conductor at the speed of light! When I suggested that this wasn’t the case, he replied that that is what he learned from text books back in the 30’s.
I hadn’t long since graduated from university with majors in Physics and Chemistry and had the maths at my fingertips. I went to the board at the front of the room, started from basic principles, considering the crystal lattice nature of metals and derived an equation, filling the board with mathematics, that showed the drift velocity of an electron in a conductor was only a couple of metres per second. He understood and also realized that the electric current effect travelled at the speed of light due to the speed of the electric field in the conductor. I also thought at the time that I wished that when I turned 75, I’d understand such mathematics and would be able to change my mind about something I had believed for so long.
Why am I talking about electrons? Well, recently I had a beer or three with some ex-Radschool graduates, a couple of whom I had actually taught. Somehow, electrons became the topic of conversation…that’s beer for you! And we had a difference of opinion, because the ex-techos were not far removed in their beliefs to that of old teacher I once knew.
At Radschool we talked about electron current. These
guys had come to Radschool for the conversion course from mechanic to
technician and didn’t have the benefit of doing Radio’s 3, 4 and 5 in the
mechanics’ course. These new courses covered semiconductors where a
different kind of current was revealed, ‘hole’ current. They had learned
that current went from negative to positive, and so it does with electron
current, well not really.
Michael Faraday probably knew more about electricity than any of us will ever know, and he did not even know that atoms existed, let alone electrons. James Clerk Maxwell, who revolutionized our knowledge of electricity and magnetism, knew nothing of electrons. Indeed, they knew of electric current, which they treated as a fluid and it went from positive to negative. Today this is called ‘conventional’ electric current. Universities do not bother with electron current when teaching Physics. The most important thing to understand is that we talk in ‘models’. There is the electron current model etc…. So what is an electron?
I don’t really know what an electron is. I can describe it mathematically, but not physically. It has no physical dimensions, that is, it has zero size! It has properties that can be measured, such as mass, charge and quantum mechanical spin number. Sounds crazy? Well yes, even though the electron has no physical dimensions, it behaves as if it spins and this can be detected in a magnetic field. But this ‘spin’ is not physical spin as such, it is a term physicists made up to explain some weird properties. For example, an electron has to ‘spin’ twice on its ‘axis’ for us to observe the same state or ‘appearance’. So we say that it has spin of plus or minus (depending on the direction of spin) one half. For simplicity we say that electrons either spin up or spin down. This is the particle model of the electron. This model can be shown to be correct and it can be shown to be false! (SEE!!! makes a lot of sense this when you get into it - tb)
Back to the beer or three discussion. The belief held
was that electrons in a metal were fixed in place with the ‘parent’ atom
and were bumped on, just like the balls in a Newton’s Cradle, hence
producing the flow of electrons and that explained why the drift velocity
was so slow
For one thing, electrons are never stationary. They always move. Why do they have to move? Because of Wolfgang Pauli, an Austrian physicist, utterly useless and worse in a laboratory, but a brilliant theorist. While Pauli was around, physicists were careful what they published, because he read everything and could be very caustic in his remarks, such as ‘this is so bad, it isn’t even wrong!’ What is called the Pauli Exclusion Principle in effect says, among other things, that no two electrons can have the same energy.
Hence electrons near each other must keep moving and in a metal could be said to behave like particles of a gas moving throughout the metallic crystal lattice.
So why do I keep ranting on about models? Because that
is all we can use, because we can never see an electron! Or an atom for
that matter. We can only see a very fuzzy impression. What about more
powerful microscopes? Well, microscopes need to use waves (particles) to
‘see’ something. You radar guys will know
The Heisenberg Uncertainty Principle ensures that we cannot ever see an electron with any precision. The principle says that we cannot tell both the position of a particle and its motion with precision at the same time. Let’s try a thought experiment:
You are in a billiard room which is in total darkness. At the end of the table is a red ball, and you have a bag full of white balls. Find the red ball. OK, so you roll a white ball down the table and if it hits the red ball, you hear a click. Got it! No, now you have moved the red ball and you know where it was, but not where it is.
You get higher precision with a sharp, loud click from
rolling the white ball faster (with higher energy and
Of course, on the macro scale we don’t notice our effect on the subject. Although, I suspect that any of you who have had a biopsy taken for suspected prostate cancer will know the effect of a measuring device, especially in the macro scale!
I suppose it is simpler just to see electrons as little billiard balls carrying a charge, which renders them prone to force in an electric field. Remember, that Nicola Tesla, who we must be forever grateful for AC generators and electricity supplies, didn’t need to know anything about electrons.
Just a few follow-up points:
If you haven’t gone to sleep by now, just a question for you:
What is an electric field anyway? Remember those lines you used to draw or saw in text books. It’s all rubbish you know; they don’t exist. That’s right, the lines are part of a useful model we use to explain some observable phenomena.
|
|
|
Van der Merwe had never been out of South Africa before and was visiting Bondi Beach, Australia . He spotted a long line of black dots out in the water and said to an Aussie, who was sitting close by, "What are all those little black things out there?" "They're buoys," said the Aussie. "Boys?!" replied Van der Merwe. "What are they doing out there?" "Holding up the shark net," the Aussie told him. "Great country this!" said Van der Merwe , deeply impressed. "We'd never get away with that at home!" |
|
|
|

 Electrons
will move from areas of low potential to areas of high potential. That can
be from negative to negative or positive to positive, namely from -100
volts to -50 volts and from +50 volts to +100 volts, or even from -50
volts to +50 volts. In a fluorescent tube, there is current in both
directions. OK, OK, simple stuff, but definitions become important after a
few beers.
Electrons
will move from areas of low potential to areas of high potential. That can
be from negative to negative or positive to positive, namely from -100
volts to -50 volts and from +50 volts to +100 volts, or even from -50
volts to +50 volts. In a fluorescent tube, there is current in both
directions. OK, OK, simple stuff, but definitions become important after a
few beers. 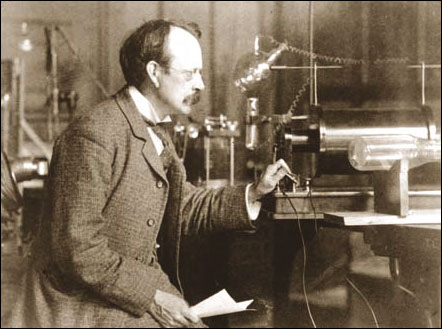 At
the Cavendish Laboratories in Cambridge University, at the end of the 19th
century, J.J. Thomson discovered the electron particle and eventually
received the Nobel Prize. His son, G.P. Thomson, years later, showed that
the electron was not a particle, but was a wave, for which he was awarded
the Nobel Prize. Both men were correct. I have done many experiments in
laboratories showing both to be correct. This means that there were two
models of the electron, up to that stage anyway. This is all part of the
concept in Physics called ‘wave/particle duality’. All particles can be
considered to be waves and vice-versa, depending on how they are observed.
Light and radio waves follow the same rule.
At
the Cavendish Laboratories in Cambridge University, at the end of the 19th
century, J.J. Thomson discovered the electron particle and eventually
received the Nobel Prize. His son, G.P. Thomson, years later, showed that
the electron was not a particle, but was a wave, for which he was awarded
the Nobel Prize. Both men were correct. I have done many experiments in
laboratories showing both to be correct. This means that there were two
models of the electron, up to that stage anyway. This is all part of the
concept in Physics called ‘wave/particle duality’. All particles can be
considered to be waves and vice-versa, depending on how they are observed.
Light and radio waves follow the same rule.  compared to the speed of the current effect.
compared to the speed of the current effect.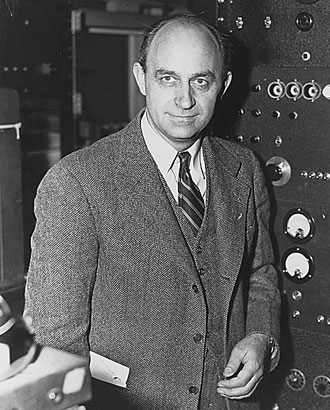 Enrico
Fermi (Nobel Laureate) devised a set of mathematical principles to explain
electron behaviour, after watching the motions of ants on a nest at a
picnic. Despite the complexity of ‘Fermi gas’ theory, it is only a model
which explains some features of metals. Later models involve a special
system that is not the simple length, breadth, height that we are used to
in our everyday lives. Wave vector space is used to describe a model using
Brillouin Zones. These are three dimensional surfaces that surround atoms
in a crystal lattice, down which the electrons move. These are zones of
minimum energy. What are the two great driving forces in nature? Hunger
and sex? Well, maybe no, the drives to minimum energy and maximum chaos.
Hold your beer glass over a tiled floor and allow it to drop. It will
change into a state of minimum energy and maximum chaos, shattered to
pieces and a mortifying waste of beer! The reverse process will not occur.
(SEE!!! - told you, makes a heap of sense
- tb)
Enrico
Fermi (Nobel Laureate) devised a set of mathematical principles to explain
electron behaviour, after watching the motions of ants on a nest at a
picnic. Despite the complexity of ‘Fermi gas’ theory, it is only a model
which explains some features of metals. Later models involve a special
system that is not the simple length, breadth, height that we are used to
in our everyday lives. Wave vector space is used to describe a model using
Brillouin Zones. These are three dimensional surfaces that surround atoms
in a crystal lattice, down which the electrons move. These are zones of
minimum energy. What are the two great driving forces in nature? Hunger
and sex? Well, maybe no, the drives to minimum energy and maximum chaos.
Hold your beer glass over a tiled floor and allow it to drop. It will
change into a state of minimum energy and maximum chaos, shattered to
pieces and a mortifying waste of beer! The reverse process will not occur.
(SEE!!! - told you, makes a heap of sense
- tb)  that a short wavelength radar pulse will give better definition of the
target that a long wavelength pulse. The shorter the wavelength, the
better distinction between targets. The rule of thumb is that to get good
definition of the target, the wavelength of the radiation should be
similar to the target being ‘probed’. An atom is about one angstrom in
size, that is 10 to the minus 10 metres. That is the wavelength of an
X-ray and such radiation is used in determining the crystal structure of
matter. But, you still cannot see detail of the atom. A proton/neutron is
about one hundred-thousanth the size of an atom. So even shorter
wavelengths have to be used to ‘see’ particles in the nucleus. Electrons
have zero size!
that a short wavelength radar pulse will give better definition of the
target that a long wavelength pulse. The shorter the wavelength, the
better distinction between targets. The rule of thumb is that to get good
definition of the target, the wavelength of the radiation should be
similar to the target being ‘probed’. An atom is about one angstrom in
size, that is 10 to the minus 10 metres. That is the wavelength of an
X-ray and such radiation is used in determining the crystal structure of
matter. But, you still cannot see detail of the atom. A proton/neutron is
about one hundred-thousanth the size of an atom. So even shorter
wavelengths have to be used to ‘see’ particles in the nucleus. Electrons
have zero size!  momentum),
but you will have moved the red ball further. OK, roll the white ball down
the table very slowly so that it doesn’t move the red ball. Sorry, there
will be no click and you will be none the wiser. In other words, the
process of making a measurement, changes the thing you are measuring. A
simple example of this is taking the temperature of a cup of hot tea.
Immerse the bulb of the thermometer, the mercury gets hotter and expands,
but now you have taken heat out of the tea and have therefore changed its
temperature.
momentum),
but you will have moved the red ball further. OK, roll the white ball down
the table very slowly so that it doesn’t move the red ball. Sorry, there
will be no click and you will be none the wiser. In other words, the
process of making a measurement, changes the thing you are measuring. A
simple example of this is taking the temperature of a cup of hot tea.
Immerse the bulb of the thermometer, the mercury gets hotter and expands,
but now you have taken heat out of the tea and have therefore changed its
temperature.